Em 1959, Tom Lehrer decidiu cantar o nome de todos os 101 elementos químicos conhecidos até então, sobre a melodia da música “I Am The Very Model Of A Modern Major-General (The Major-Generals Song)”, da “opera buffa” The Pirates of Penzance, composta por Sir Arthur Sullivan em 1879. Segue abaixo a gravação original de Lehrer, seguida de uma apresentação mais recente da música original de Sullivan:
The element song (Tom Lehrer 1959)
There’s antimony, arsenic, aluminum, selenium,
And hydrogen and oxygen and nitrogen and rhenium,
And nickel, neodymium, neptunium, germanium,
And iron, americium, ruthenium, uranium,
Europium, zirconium, lutetium, vanadium,
And lanthanum and osmium and astatine and radium,
And gold and protactinium and indium and gallium, (gasp)
And iodine and thorium and thulium and thallium.
There’s yttrium, ytterbium, actinium, rubidium,
And boron, gadolinium, niobium, iridium,
And strontium and silicon and silver and samarium,
And bismuth, bromine, lithium, beryllium, and barium.
There’s holmium and helium and hafnium and erbium,
And phosphorus and francium and fluorine and terbium,
And manganese and mercury, molybdenum, magnesium,
Dysprosium and scandium and cerium and cesium.
And lead, praseodymium and platinum, plutonium,
Palladium, promethium, potassium, polonium,
And tantalum, technetium, titanium, tellurium, (gasp)
And cadmium and calcium and chromium and curium.
There’s sulfur, californium and fermium, berkelium,
And also mendelevium, einsteinium, nobelium,
And argon, krypton, neon, radon, xenon, zinc and rhodium,
And chlorine, carbon, cobalt, copper, tungsten, tin and sodium.
These are the only ones of which the news has come to Harvard,
And there may be many others but they haven’t been discovered.
I am the very model of a modern major-general
Gilbert and Sullivan’s raucous operatic tale is captured in all its fun and glory in this production, recorded live at Central Park’s Delacorte Theater.
Como complemento, segue abaixo uma gravação de Lehrer cantando a música em uma apresentação em Copenhagen (Dinamarca), em 1967:
Tom Lehrer – The elements (Copenhagen 1967)
The melody to The Elements is I Am The Very Model Of A Modern Major-General (The Major-Generals Song) from the opera buffa The Pirates of Penzance. It was composed by Sir Arthur Sullivan, and it was first premiered in New York on December 31st 1879. The original libretto for the opera was written by Sir William Schwenck Gilbert. Recording date: September 5th 1967 Location: Falkonercenteret, Copenhagen, Denmark Format: Most probably Ampex Quadruplex PAL 4:3 Status: A rare recording indeed Storage: Most probably Sony Digital Betacam and in a digital format Production and preservation: Danmarks Radio (DR) in Denmark More HERE:
Apresento a versão abaixo pela qualidade das imagens representando os elementos:
The Elements Song
spicytito15
Mais recentemente, Dennis Nowicki regravou a música de Lehrer, em andamento bem mais lento, e atualizando-a para os 118 elementos atualmente conhecidos:
Periodic Table of Elements Song – All 118 Elements
Satirist Tom Lehrer’s Elements song updated to the current 118 Elements. It’s a bit slower to help with easier memorization, and humbly performed by Dennis Nowicki.
There’s antimony, arsenic, aluminum, selenium,
and hydrogen, and oxygen, and nitrogen, and rhenium,
and nickel, neodymium, neptunium, germanium,
and iron, americium, ruthenium, uranium,
Europium, zirconium, lutecium, vanadium,
and lanthanum, and osmium, and astatine, and radium
and gold, protactinium, and indium, and gallium,
and iodine, and thorium, and thulium, and thallium.
There’s yttrium, ytterbium, actinium, rubidium
and boron, gadolinium, niobium, iridium,
and strontium, and silicon, and silver, and samarium,
and bismuth, bromine, lithium, beryllium, and barium.
There’s holmium, and helium, and hafnium, and erbium,
and phosphorus, and francium, and fluorine, and terbium,
and manganese, and mercury, molybdenum, magnesium,
dysprosium, and scandium, and cerium, and cesium,
and lead, praseodymium, and platinum, plutonium,
palladium, promethium, potassium, polonium,
and tantalum, technetium, titanium, tellurium,
and cadmium, and calcium, and chromium, and curium.
There’s sulfur, californium, and fermium, berkelium,
and also mendelevium, einsteinium, and nobelium,
and argon, krypton, neon, radon, xenon, zinc, and rhodium,
and chlorine, carbon, cobalt, copper, tungsten, tin, and sodium.
There’s seaborgium, meitnerium, nihonium, and bohrium,
and hassium, lawrencium, dubnium, livermorium,
tennessine, oganneson, copernicium, flerovium,
Rutherfordium, darmstadtium, roentgenium, moscovium.
118 elements, I think we’ve got these covered
But, who knows, there may still be more that are yet undiscovered.
Em 2013, o canal ASAP Science publicou uma nova música, dessa vez listando os elementos por ordem crescente de número atômico, sobre um acompanhamento de Can Can. Segue abaixo a versão original, e dois vídeos particularmente bem ilustrados (por Andy Tsang e Engineered Labs):
The New Periodic Table Song
AsapSCIENCE – Tema
2013
The Most Colorful (and Cute) Periodic Table (ASAPSCIENCE Song in 2021)
Andy Tsang
The Periodic Table Song with real elements
Engineered Labs
Encontrei no canal KLT uma música impressionante de mais de 47 minutos, na qual cada elemento se apresenta brevemente em forma de rap cantado:
Periodic Table of Elements Song
KLT
Também fiquei bem impressionado com esta música de David Newman, que lista todos os elementos em ordem crescente de número atômico:
These Are The Elements (Periodic Table Song, in order)
David Newman
2011
E por fim, seguem abaixo a versão original (gravada no disco Here Comes Science, de 2009) e uma versão acústica (gravada em 2010) da música “Meet the Elements”, da banda They Might Be Giants:
They Might Be Giants – Meet The Elements (oficial TMBG video)
TMBGkids
Meet The Elements (Acoustic Version) – They Might Be Giants 26 June 2010
astralbee
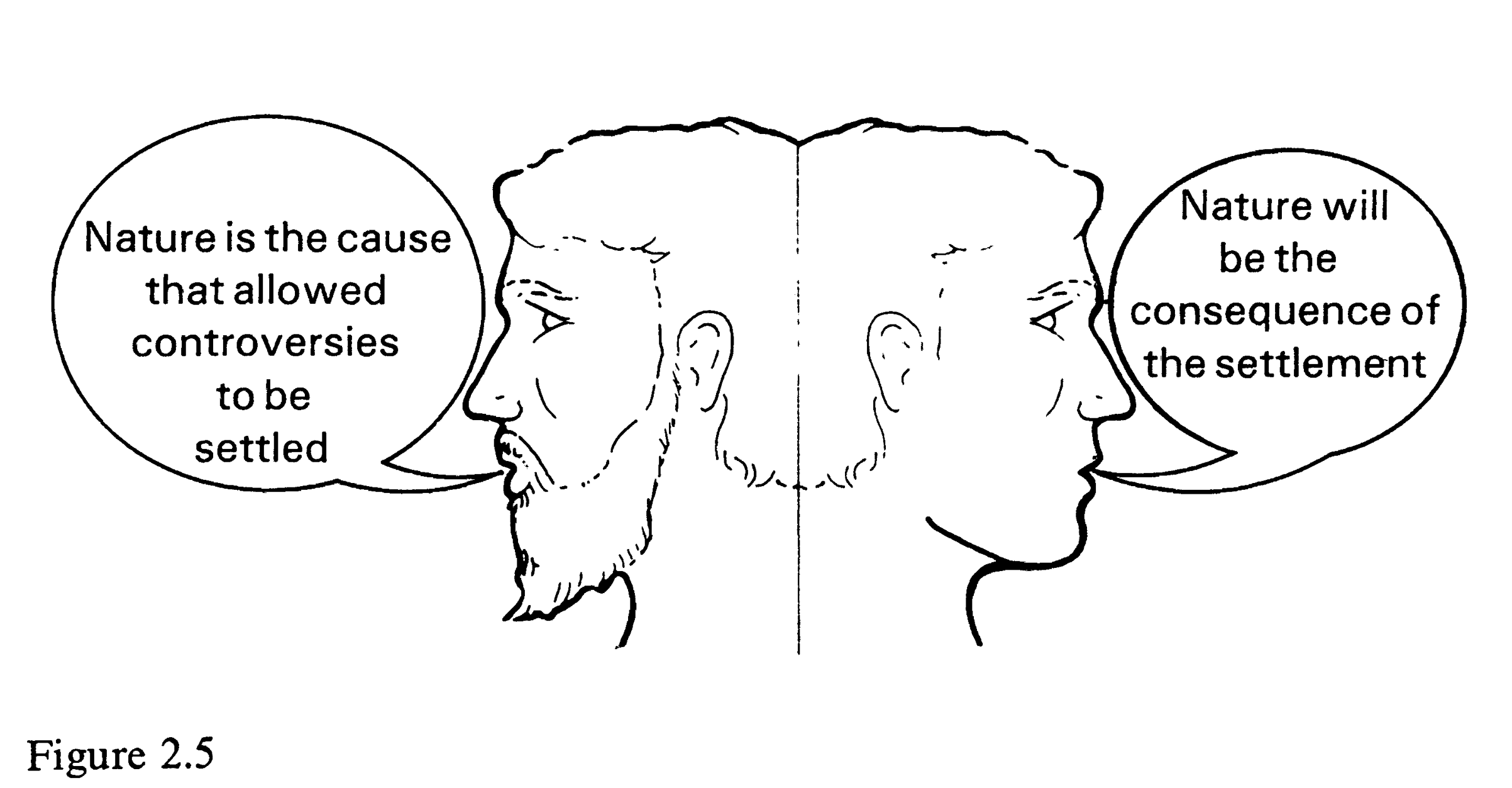 (Latour 1987:99 Fig. 2.5)
(Latour 1987:99 Fig. 2.5)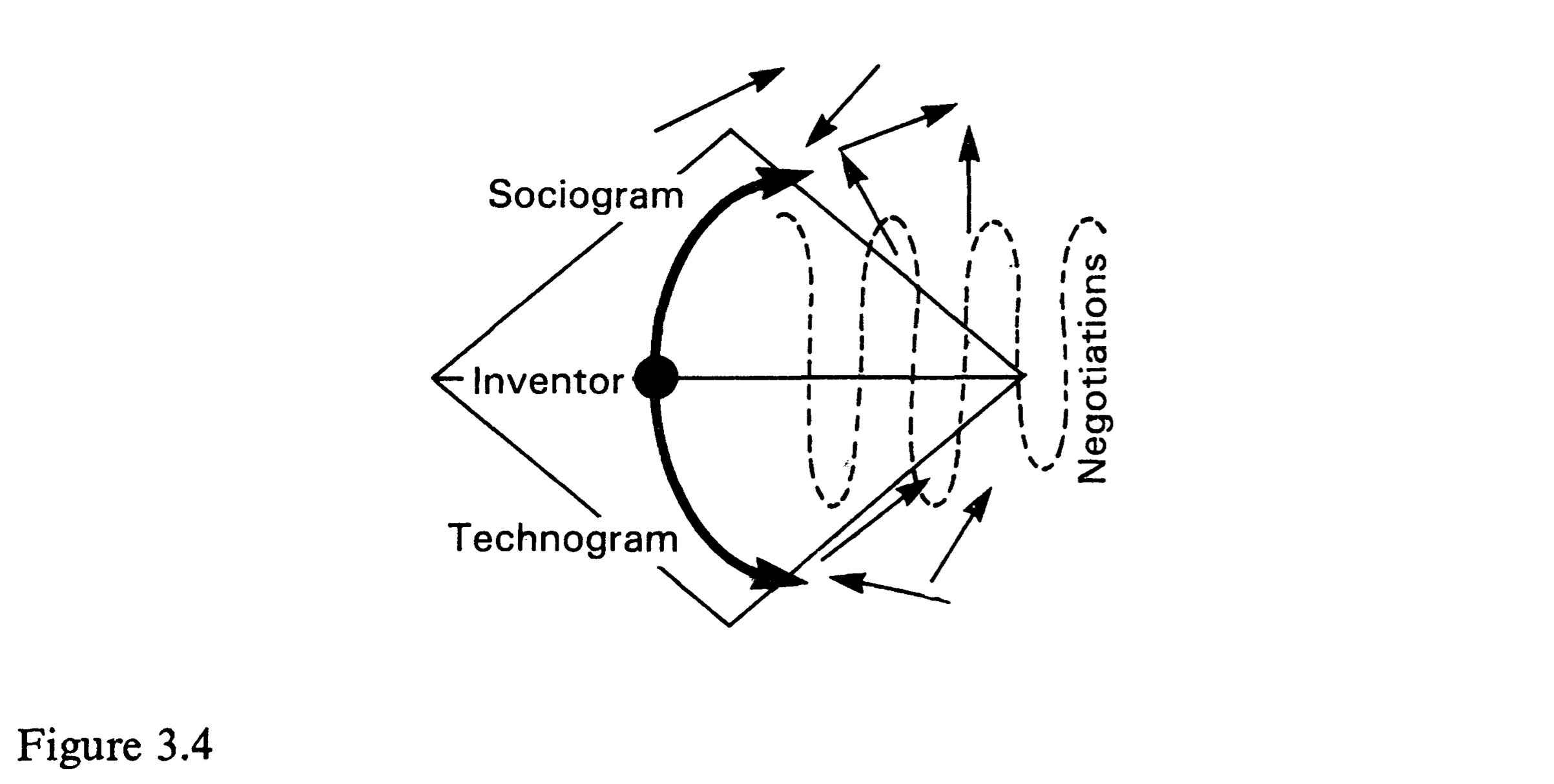 (Latour 1987:139 Fig. 3.4)
(Latour 1987:139 Fig. 3.4)